
Citations

Related Articles:
Grube, A.; Kock, M. Org. Lett. 2006, 8, 4675-4678.
Kock, M.; Grube, A.; Seiple, I. B.; Baran, P. S. Angew. Chem., Int. Ed. Engl. 2007, 46, 6586-6594.
Main article:
Lanman, B. A.; Overman, L. E.; Paulini, R.; White, N. S. J. Am. Chem. Soc. 2007, 129, 12896-12900.
This article discussed the synthesis of marine alkaloid palau'amine. This compound has received much attention in the scientific community because of its effects of the immune system and challenging molecular structure. It relates to the group's journal article in terms of similar reagents used in the synthesis reaction. One reagent is DMAP which is a catalyst used in esterifications with anhydrides. In the reaction to form compound 19, it is used to add Boc as a protecting group to nitrogen. NaBH4 is used as a reducing agent in both articles to form an alcohol from a ketone. Tetrahydrofuran (THF) is used as an aprotic solvent in which many of the reactions from both articles take place.
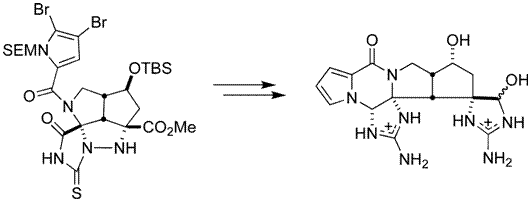
This article was cited by:
Bultman, M. S.; Ma, J.; Gin, D. Y. Angew. Chem. Int. Ed. 2008, 47, 6821-6824.
-Boc is also used as a protecting group and DMAP is used as a catalyst for esterification of an anhydride.
Arndt, H.; Riedrich, M. Angew. Chem. Int. Ed. 2008, 47, 4785-4788.
-NaBH4 is used as a source of hydrogen for a carbonyl group. DMAP is used a catalyst for esterification reaction to add Ac2O.
Zancanella, M. A.; Romo, D. Org. Lett. 2008, 10, 3685-3688.
-DMAP was also used as a catalyst for esterification of an anhydride.
