Molecular Structures of Buried Interfaces in Polymer Thin Films
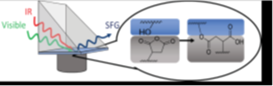
Multilayer polymer films are extensively used for the transport and storage of goods in many industries, offering a reliable, sanitary, and inexpensive solution to meet the demands of increasingly complex applications and environments. Each film contains layers with different polarity; therefore it is important to introduce reactive functionalities to nonpolar layers so that they can be well adhered to polar layers.
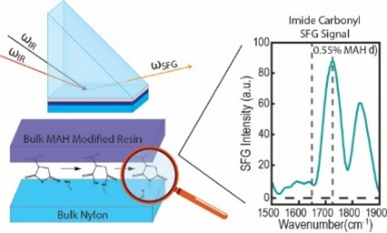
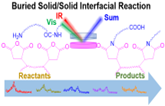
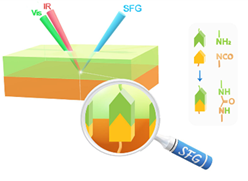
We are using SFG to study interfacial chemical reactions between different layers in situ nondestructively. Both model systems with spin coated films and commercial casting films and blown films are investigated.
We have successfully elucidated chemical reaction mechanisms at buried interfaces in multilayer polymer films, followed reaction kinetics, and deduced interfacial activation energy. We continue to study interfacial chemical reactions of new polymer films.
We are also studying interfacial interactions between various coatings and polymer films to understand molecular mechanisms of release coating tapes.